I have finished compiling the data on batch culture propagations conducted with five of the Brettanomyces strains. The following two graphs contain data from the standardized propagation method described in the previous post using 12 °Plato (1.048 gravity) wort as the growth medium. Some variation between growth patterns can be seen along with what appears to be step-like growth until the stationary phase is reached after a period of 168 to 192 hours. More information on Propagation and Batch Culture Growth can be found by following the link.
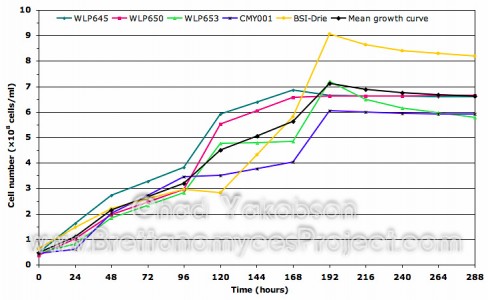
Growth curve for five strains of Brettanomyces during semi-aerobic batch culture.
Cultures were grown in 500 ml of wort substrate over a 288-hour
period at 28°C with 80-rpm agitation. Viability was taken into
account to reflect the actual cell number.
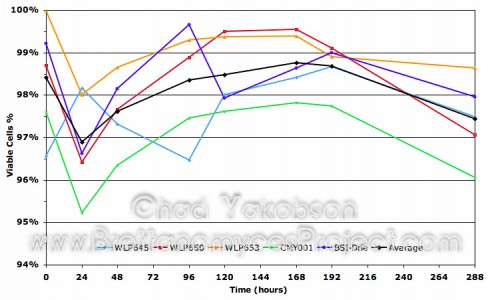
Daily percentage of cells which appeared viable after using the
methylene blue staining technique. Cultures were grown in
500ml of wort substrate over a 288-hour period at 28°C
with 80-rpm agitation.
*Methylene Blue staining technique… The use of methylene blue to stain cells in order to determine viability proved difficult with Brettanomyces spp. I found often that cells which were viable would take up the methylene blue into the vacuoles, giving a false positive. Some would take it into the cell cytoplasm but not into the vacuole. The dye would only appear near the tips giving the cells two blue dots at each end. I have spoke with other brewers who have had trouble using methylene blue to yield positive results when doing viability checks on Brettanomyces strains. I would say from the lab experience I had and daily cell counts that an alternative method would be advantageous as I don’t believe methylene blue yields completely accurate results.

any thoughts on measurement error?
hmm… To be honest I would say measurement error would be small to negligble. The propagations were done multiple times in duplicate with different starting cell concentrations and the growth was nearly always the same. If ever a cell count seemed out of the ordinary it was redone and if the re-count did not match up (which almost never happened) I would take a thrid cell count and then throw out the odd count and take an avarage. The sampling method is always identicle and the cells are so small I didn’t get clumps of cells as you do with Saccharomyces.
As for the methylene blue stain, there isn’t much more I can say. It is an industry and academic standard yet is by no means perfect, so best judgement must be used. I personally feel my viability counts were acurate as my numbers match common literataure for Saccharomyces spp. and what we have here at Odell’s with our house yeast strain during propagation.
I have some more data which I’ll post showing the difference the growth media makes on cell growth. That proved to make a visible difference.
Chad
I recommend trying a mixture of fluorescein diacetate / propidium iodide under a fluorescence microscope for vital staining. A literature search will turn up the details.